DISCOVER MORE
DISCOVER MORE  DISCOVER MORE
DISCOVER MORE  DISCOVER MORE
DISCOVER MORE with your scientific articles

11-14 November 2024

Regulatory affairs
For medical devices and IVDs

Quality
management systems
For medical devices and IVDs

Medical writing and scientific communication
For the life science world
Not sure which of our services is right for you?
Select your area of interest and orientate yourself using our guide.
SSCP: the document that makes a medical device’s safety visible. It’s not just a technical file—it’s a tool for public…
Although Regulation (EU) 2017/745 on medical devices (MDR) does not explicitly mention surveys, they are recognized in applicable standards and…
PMCF surveys are effective tools for collecting post-market clinical evidence quickly, sustainably, and in compliance with the MDR, when well…
IEC 62366-1 provides a structured approach to evaluating the usability of medical devices.Reducing use-related errors means enhancing both safety and…
Discover how to build an Oscar-worthy clinical evaluation for your medical device: a solid CER, robust data, and an expert…
The European Union is accelerating its efforts in biotechnology and biomanufacturing with an integrated strategy aimed at strengthening innovation, sustainability,…
The bibliography of a clinical protocol serves to demonstrate that the protocol is built on a solid foundation of existing…
The introduction of an article is meant to…introduce what has been done! But what structure can we give it?
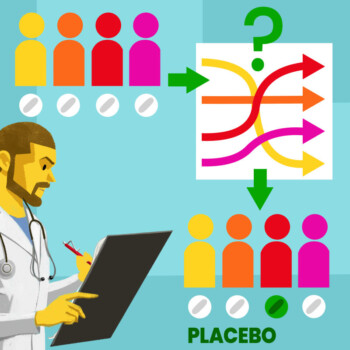
Randomization is a statistical procedure that, when applied in clinical studies, assigns participants to different treatment groups randomly.
Inspection verification is a fundamental tool that companies can—and must—adopt to assess whether their Quality Management System (internal audit) or…
The risk management activity should be entrusted to a qualified team formed for this purpose, usually consisting of at least…
In any clinical study, researchers often encounter datasets with missing observations, commonly referred to as “missing data.”
According to the MDR Regulation, the technical documentation must be complete, well-structured, and contain sufficient information to demonstrate that the…
The data recording system aims to collect the results of all activities performed, all checks conducted, and the results obtained,…
The written presentation of a product can be defined as any activity aimed at describing and/or illustrating the product in…
The ISO 13485 standard establishes that the organization must conduct internal audits at planned intervals: it is good practice to…
I corsi di formazione di Clariscience
Le tematiche principali e più attuali relative agli affari regolatori e alla qualità di dispositivi medici e IVD, e al medical writing e alla comunicazione scientifica per l'intero mondo life science.
Ancora nessun corso in calendario
Try Raqa Underground!
RAQA Underground is the interactive map that visually narrates regulatory affairs and quality assurance in the world of medical devices.