News & Stories
Scientific Communication
The bibliography of a clinical protocol serves to demonstrate that the protocol is built on a solid foundation of existing…
The introduction of an article is meant to…introduce what has been done! But what structure can we give it?
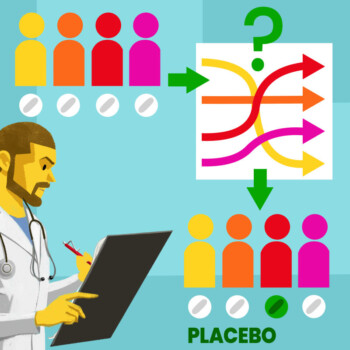
Randomization is a statistical procedure that, when applied in clinical studies, assigns participants to different treatment groups randomly.
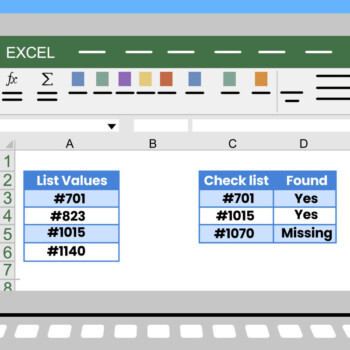
In any clinical study, researchers often encounter datasets with missing observations, commonly referred to as “missing data.”
The written presentation of a product can be defined as any activity aimed at describing and/or illustrating the product in…
The regulatory review of a protocol or a clinical study report is a crucial step in the development and commercialization…
Planning clinical research is a well-structured process aimed at transforming an idea into clear, coherent, reliable, presentable, and contestable data.
Slides are an important tool for helping the audience visualize the logical flow of the oral presentation, while also engaging…